Mechanistic Insight into the Copper-Catalyzed Phosphorylation of Terminal Alkynes: A Combined Theoretical and Experimental Study
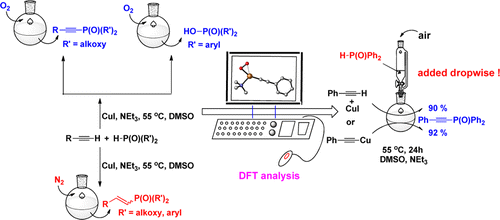
The reaction mechanism of copper-catalyzed phosphorylation of terminal alkynes under different conditions has been investigated experimentally and theoretically. The important role of dioxygen has been elucidated, including the formation of η1-superoxocopper(II), η2-superoxocopper(III), μ-η2:η2-peroxodicopper(II), and bis(μ-oxo)dicopper(III) complexes. More importantly, the proton transfer from the dialkyl phosphonate (in the form of phosphite) to the bridging oxygen atom entails the migration of the deprotonated phosphonate to the terminal alkyne, leading to the formation of a C–P bond with an activation barrier of only 1.8 kcal/mol. In addition, a particularly stable six-centered dicopper(I) species is formed with the migration of both of the Ph2P(O) groups from the copper atoms to the oxygen atoms of the bis(μ-oxo) bridge, explaining the experimental observation that secondary phosphine oxides can be oxidized to the phosphinic acids. Thus, the diphenylphosphine oxide was added to the reaction mixture dropwise to minimize the concentration during the reaction course. Gratifyingly, the coupling product was generated almost quantitatively when the reaction was completed.
