Nature Chemistry



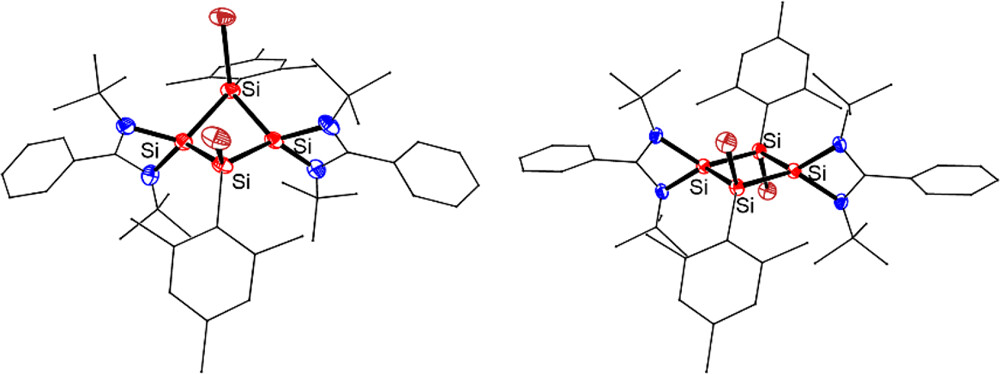
The synthesis, structures, and reactivity of the first neutral 2π-aromatic Si4 rings [LSiSiAr(X)]2 (3: X = Br; 4: X = Cl; L = PhC(NtBu)2, Ar = 2,4,6-Me3C6H2) were described. Compounds 3 and 4 were obtained by 1,3-halogenation of tetrasilacyclobutadiene (LSiSiAr)2 (2), which was prepared by the reductive cross-coupling of trisilane (ArSiCl2)2SiHAr with two equiv of chlorosilylene LSiCl.

Complexes with aromaticity in both the lowest singlet state (S0) and the lowest triplet state (T1) (denoted as adaptive aromaticity) are rare because according to Hückel’s and Baird’s rules, a species could be aromatic in either the S0 or T1 state in most cases. Thus, it is particularly challenging to design species with adaptive aromaticity. Previous reports on adaptive aromaticity were mainly focused on 16e metallapentalenes.

Aromaticity is an important concept in chemistry with multidimensional properties, attracting considerable attention from both experimental and computational chemists. Among various aromaticity indices, the harmonic oscillator model of aromaticity (HOMA) is a reliable aromaticity criterion with a negligible computational cost based on the geometry (bond distance). However, the HOMA parameters for organometallic aromatics are not available. Here, we develop the Os–C bond parameter of HOMA by theoretical calculations.

The photoswitching behaviors of heteroaryl azos and azobenzenes have attracted considerable interest due to their applications from material science to pharmacology. However, the use of UV light limits their application, especially in biomedicine and photopharmacology. In this work, using several aromaticity descriptors, including anisotropy of the induced current density analysis and nucleus-independent chemical shifts, we systematically investigate the relationship between anti-aromaticity and the absorption of a series of heterocyclic azos.

Species generally exhibit one-state aromaticity either in the lowest singlet state (S0) or the lowest triplet state (T1) according to the Hückel's and Baird's rules. Hence, it is rare for species exhibit two-state aromaticity in both the S0 and T1 states (termed as adaptive aromaticity), let alone adaptive σ aromaticity. Here, we report adaptive σ aromaticity in unsaturated rhenacyclopropene rings via density functional theory (DFT) calculations.
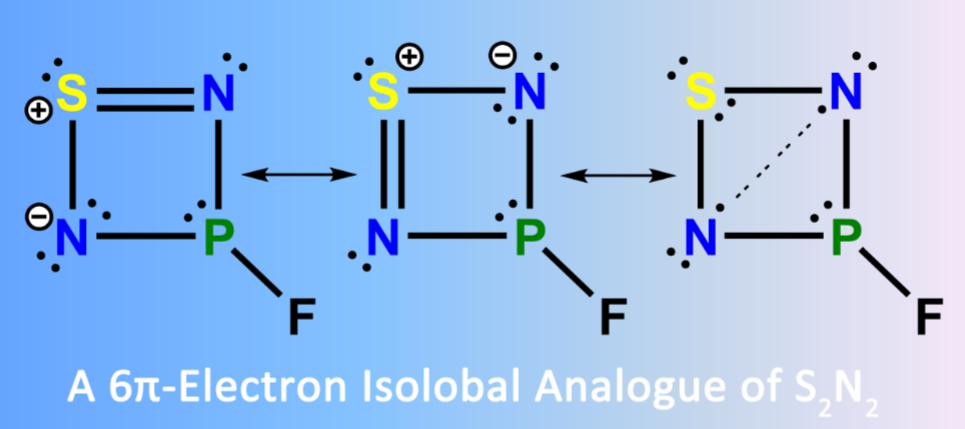
The new 6π-electron four-membered ring compound 3-fluoro-1λ2,2,4,3λ3-thiadiazaphosphetidine, FP(μ-N)2S, has been generated in the gas phase through high-vacuum flash pyrolysis (HVFP) of thiophosphoryl diazide, FP(S)(N3)2, at 1000 K. Subsequent isolation of FP(μ-N)2S in cryogenic matrices (Ar, Ne, and N2) allows its characterization with matrix-isolation IR and UV-vis spectroscopy by combination with 15N-isotope labeling and computations at the CCSD(T)-F12a/VTZ-F12 level of theory.
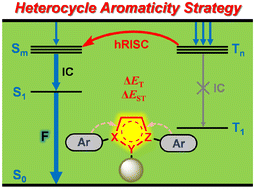
Efficiently harvesting electroluminescent triplet excitons is of great importance for practical applications of organic light-emitting diodes (OLEDs). Hot exciton materials are regarded as the up-and-coming new generation luminogens and hold unique advantages for achieving high efficiency electroluminescent devices. Therefore, exploring a new molecular design strategy for developing new hot exciton materials remains a challenging yet fascinating task so far.

The Pd-catalyzed Buchwald–Hartwig coupling reaction is important in the construction of the C-N bond due to various applications in organic synthesis. Quantum chemical calculations are widely used in understanding reaction mechanisms whereas the machine learning method is extremely popular in recognizing the relationships of data. Here, we combine density functional theory calculations with the support vector regression method to probe the origin of the higher efficiency of terphenyl phosphine ligand over the biaryl counterpart in the Buchwald–Hartwig C-N coupling reaction.

Singlet fission (SF) presents an attractive solution to overcome the Shockley–Queisser limit of single-junction solar cells. The conversion from an initial singlet state to final triplet is mediated by the correlated triplet pair state 1(T1T1). Despite significant advancement on 1(T1T1) properties and its role in SF, a comprehensive understanding of the energetic landscape during SF is still unclear.
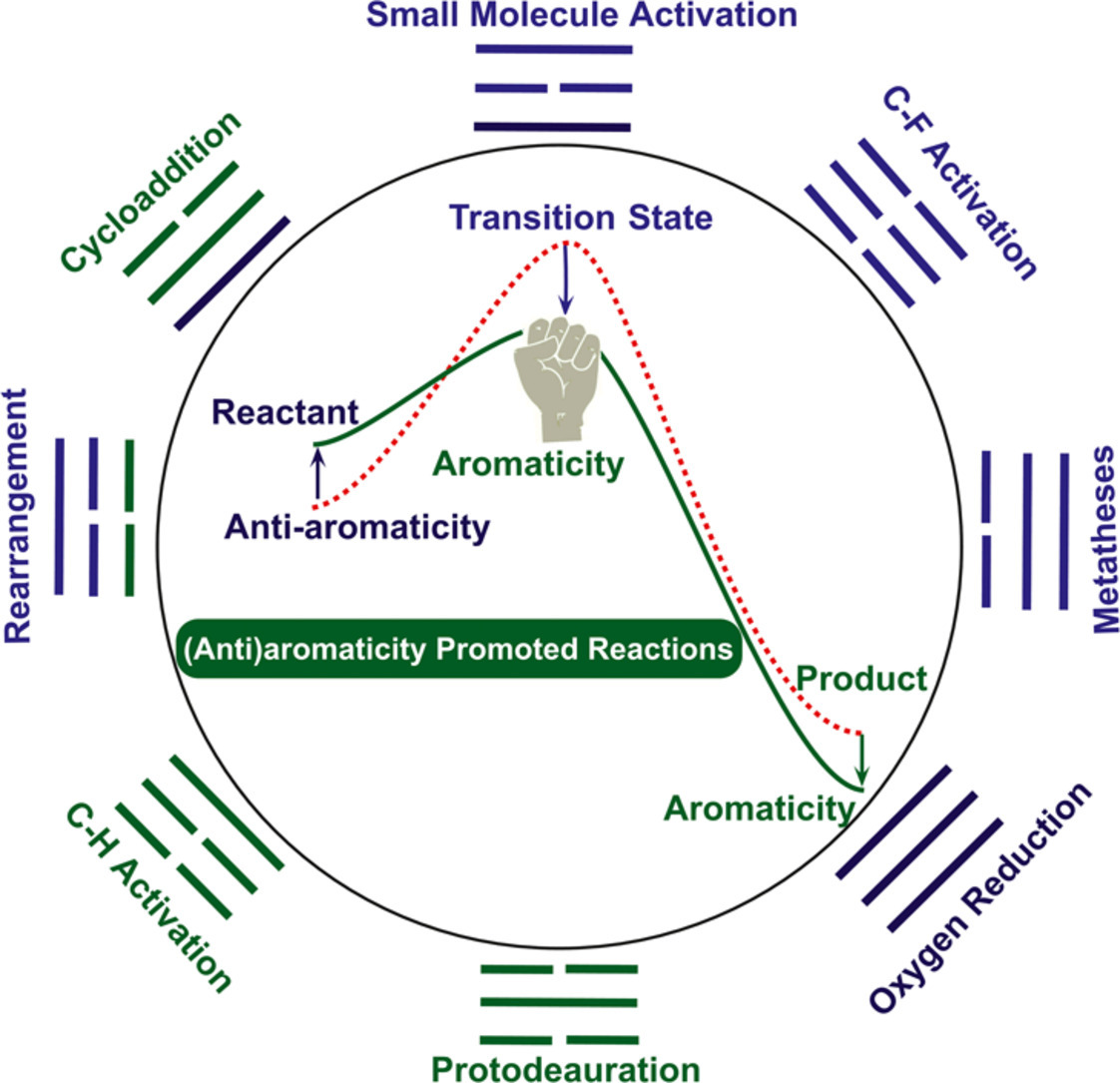
Aromaticity, in general, can promote a given reaction by stabilizing a transition state or a product via a mobility of π electrons in a cyclic structure. Similarly, such a promotion could be also achieved by destabilizing an antiaromatic reactant. However, both aromaticity and transition states cannot be directly measured in experiment. Thus, computational chemistry has been becoming a key tool to understand the aromaticity-driven reaction mechanisms.
Copyright © 2026,
Theme Originally Created by Devsaran
