Nature Chemistry



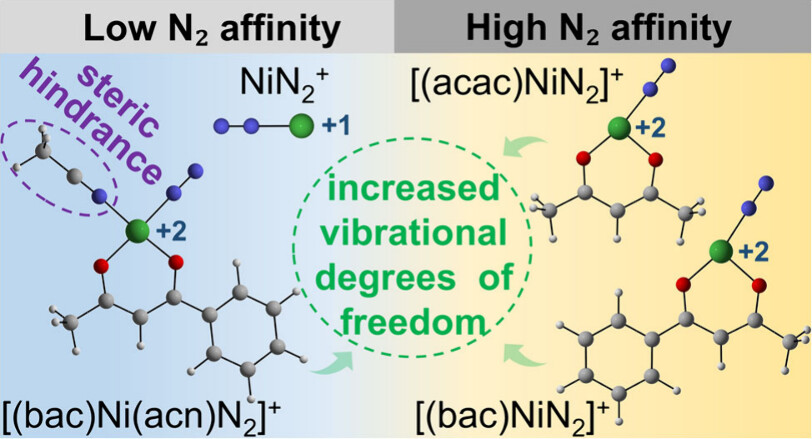
N2 adsorption on metal sites under mild conditions and its dependence on the chemical environment are crucial for understanding the fundamental mechanism and designing an efficient catalyst. Here, electrospray ionization mass spectrometry (ESI-MS) experiments reveal distinct differences in N2 binding capabilities among nickel complexes: both [(acetylacetonato)Ni]+ and [(benzoylacetonato)Ni]+ gas-phase cations exhibit high N2 adsorption efficiency, while the bare Ni+ and [(benzoylacetone)Ni(acetonitrile)]+ cations show negligible reactivity toward N2.
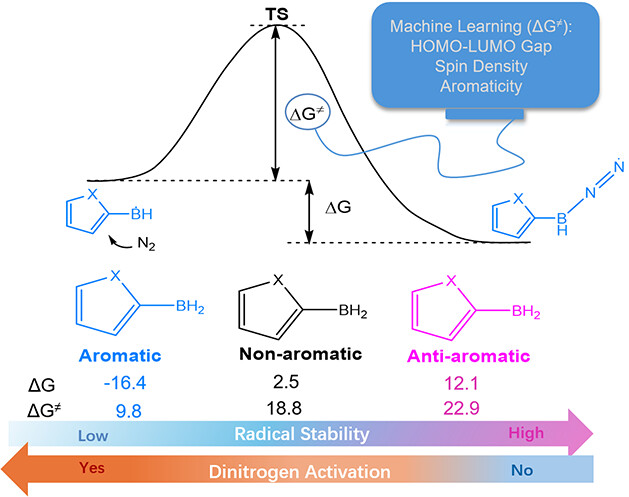
Aromaticity is one of the fundamental concepts in chemistry and generally brings additional thermodynamic stability to a compound. On the other hand, boron radicals have attracted increasing interest from both theoretical and experimental chemists due to their various applications. Here, we carry out density functional theory (DFT) calculations to explore the relationship between the (anti)aromaticity and stability of boron-centered radicals.
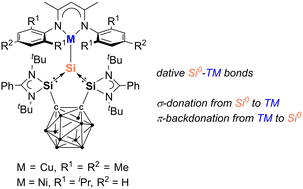
The first silylone-3d-metal complexes, LSiCu(NacNacM) (2) [L = 1,2-(RSi)2-1,2-C2B10H10, R = PhC(NtBu)2; NacNacM = HC(CMeNMes)2, Mes = 2,4,6-Me3-C6H2] and LSiNi(NacNacD) (3) [NacNacD = HC(CMeNDipp)2, Dipp = 2,6-iPr2-C6H3], are reported, resulting from the reaction of the strongly σ-donating and chelating bis(silylenyl)-ortho-carborane silylone LSi0 with [(NacNacMCu)2benzene] and [(NacNacDNi)2toluene], respectively.
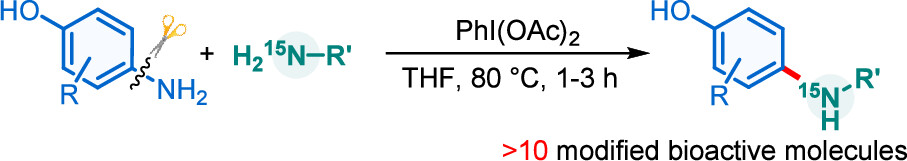
Activation of the aryl C–N bond underpins critical challenges in modern organic synthesis. Herein, the direct amination of anilines is presented via hypervalent iodine-mediated transient dearomatized phenolate intermediates, enabling selective C(aryl)–NH2 bond cleavage under mild conditions. A library of bioactive p-alkylaminophenols is synthesized in up to 85% yields within 3 h. Being used in late-stage drug diversification and mechanistic studies, this protocol offers a modular platform for complex amine construction.
Learning from nature has emerged as a promising strategy for catalyst development, wherein the remarkable performance of catalysts selected by nature over billions of years of evolution serves as a basis for the creative design of high-performance catalysts. Hydrogenases, with their exceptional catalytic activity in hydrogen oxidation and production, have been employed as prototypes for human learning to achieve better catalyst design. A comprehensive understanding of hydrogenases' structures and catalytic mechanisms is crucial to replicate and exceed their performance.
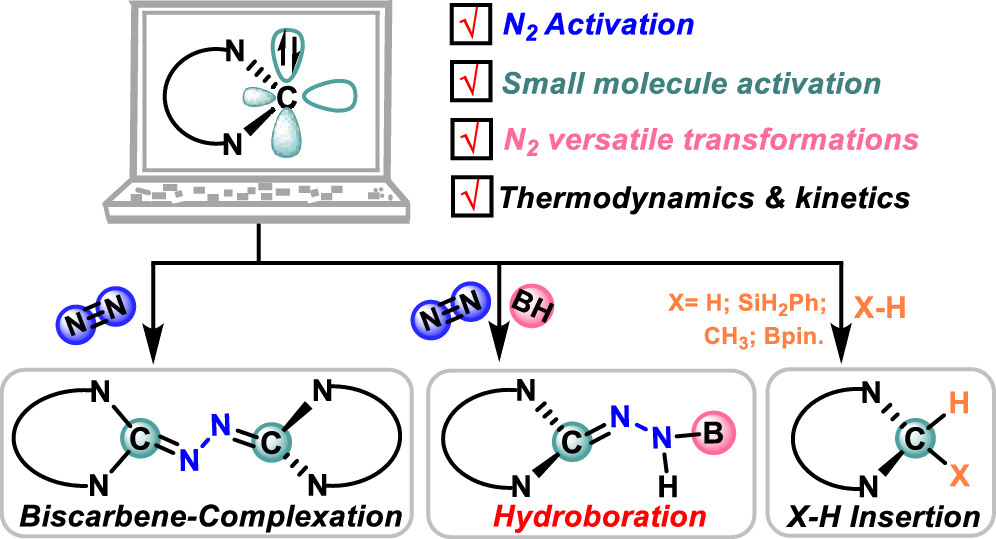
Although the carbene-catalyzed N2 fixation process had been investigated by scientists for decades prior to borylene species, the interest in the carbene-mediated N2 activation process has drawn less attention than that of borylene species in the past few years, especially unique σ0π2 carbenes. Herein, we demonstrate the important role of unique σ0π2 carbenes in the 1,1-hydroboration and bis-carbene functionalization of N2 using density functional theory calculations.
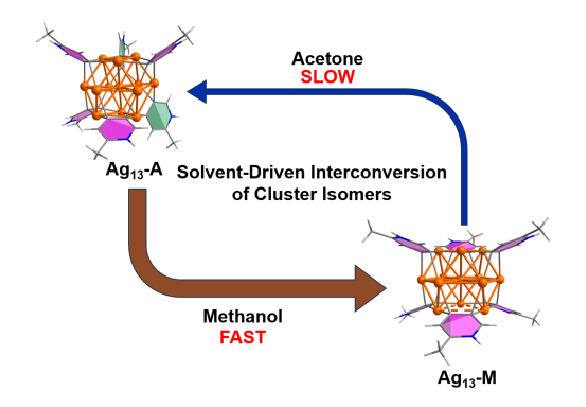
Solvent screening is pivotal in the optimization of reaction conditions for metal-catalyzed reactions. However, to date little is known about the role of solvent effects in driving in situ structural evolution of metal-containing species towards polynuclear organometallic cluster intermediates and determining their reactivity. We herein isolate two distinct thirteen-membered silver cluster isomers, Ag13-A and Ag13-M, in acetone and methanol, respectively.
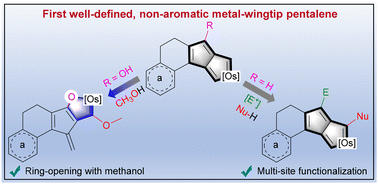
Since the concept of aromaticity was first introduced in transition metal complexes, metals have become a crucial component for modulating aromaticity, leading to a variety of structural frameworks. Initial studies successfully achieved aromatic pentalene dianions through metal-ion coordination, and recent advancements in bridgehead metallapentalenes have demonstrated the transformation of antiaromaticity into aromaticity.
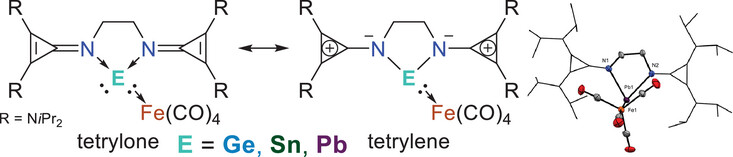
We report on the utilization of the ethylene-bridged bis[(dialkylamino)cyclopropenimine] (bisCPI) ligand, LCPI, to give access to new main-group E(II) halide complexes (E = Ge, Sn, Pb; 1, 2, 3). Subsequent reduction with Collman's reagent (Na2Fe(CO)4 • dioxane) enables the isolation of a series of zero-valent tetrylone-tetracarbonyl iron complexes, (LCPI)E(Fe(CO)4 (E = Ge (4), Sn (5), Pb (6)).
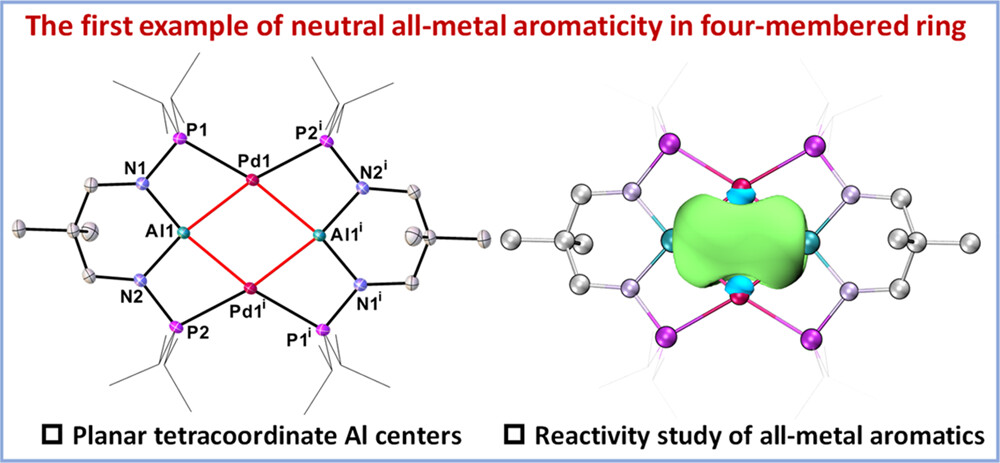
Aromaticity is a cornerstone concept in chemistry, playing a crucial role in understanding molecular stability and reactivity. Traditionally, aromaticity has been primarily associated with cyclic planar conjugated organic molecules composed solely of carbon, but it has recently expanded to include metal-containing systems. However, metal-only aromatics remain extremely scarce. Here, we present the first neutral all-metal aromatic cluster with a rhombic geometry.
Copyright © 2026,
Theme Originally Created by Devsaran
