Single-Molecule Resolved Conformational and Orbital Symmetry Breaking in Tetraphenylethylene-Based Macrocycles
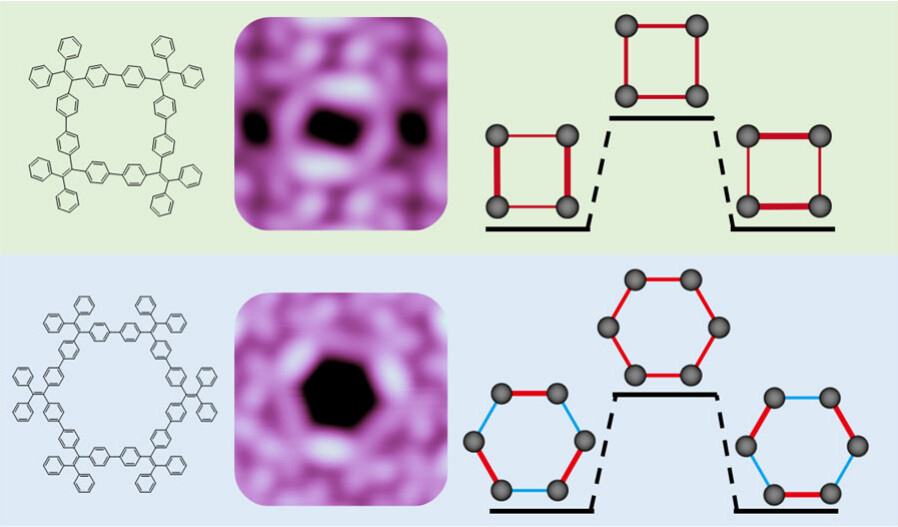
Tetraphenylethylene (TPE) is a prototype aggregate-induced emission molecule. TPE-based conjugated macrocycles exhibit unique optical properties due to their peculiar cyclic topology. Because the symmetry of macrocycles strongly affects their photophysical properties, here we report a single-molecule study of the structures and orbitals of two TPE-based macrocycles of (C26H18)4 and (C26H18)6. Using scanning tunneling microscopy and spectroscopy, we discover that both macrocycles undergo spontaneous symmetry breaking in their conformations and frontier orbitals. The computational analyses reveal that the symmetry breaking is driven by a subtle interplay of higher extended conjugation between phenyl and node carbon atoms and conformation flexibility of the macrocycles. The observed symmetry breaking in TPE-based macrocycles is expected to strongly alter their photophysical properties.
