Antiaromaticity-Promoted Radical Anion stability in α-vinyl Heterocyclics
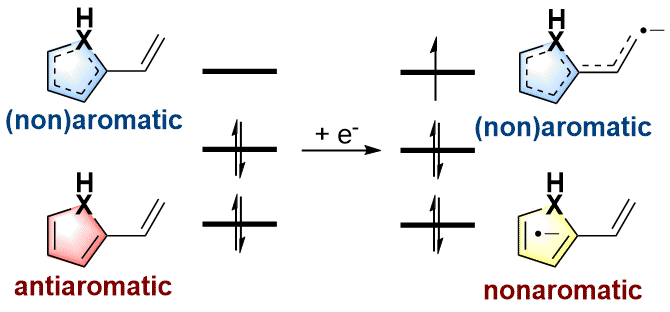
As an electron-rich species, radical anions have a wide range of applications in organic synthesis. In addition, aromaticity is an essential concept in chemistry that has attracted considerable attention from experimentalists and theoreticians. However, it remains unknown whether there is a relationship between aromaticity and thermodynamic stability of a radical anion. In this work, we demonstrate that the thermodynamically stable radical anions could be formed by the corresponding antiaromatic neutral species through density functional theory calculations. The principal interacting spin orbital analysis indicates that a large amount of unpaired electrons are populated on the heterocycles, releasing the antiaromaticity in the heterocycles and generating thermodynamically more stable radical anions according to radical stabilization energies. In other words, the stronger the antiaromaticity of the original neutral heterocyclics, the higher the thermodynamic stability of the corresponding radical anions, which is in sharp contrast to the general knowledge that aromaticity brings compounds’ thermodynamic stabilities. Our findings in this work demonstrate a novel strategy for designing thermodynamically stable radical anions.
https://pubs.rsc.org/en/content/articlelanding/2022/qo/d1qo01944a
