Antiaromaticity-Promoted Radical Anion stability in α-vinyl Heterocyclics
Submitted by Jun Zhu on Sat, 01/29/2022 - 19:53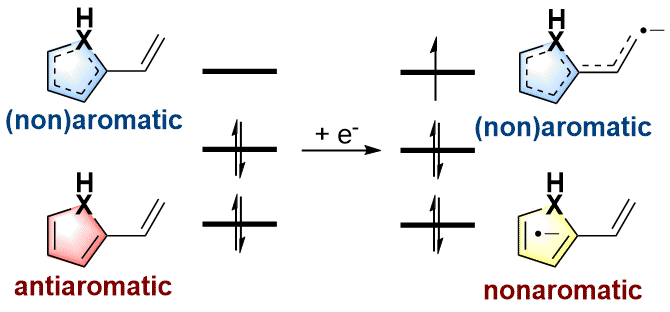
As an electron-rich species, radical anions have a wide range of applications in organic synthesis. In addition, aromaticity is an essential concept in chemistry that has attracted considerable attention from experimentalists and theoreticians. However, it remains unknown whether there is a relationship between aromaticity and thermodynamic stability of a radical anion. In this work, we demonstrate that the thermodynamically stable radical anions could be formed by the corresponding antiaromatic neutral species through density functional theory calculations.