A Conjugated Diosma-Octacyclic Complex and Its Mixed-Valence Singly Reduced State
Submitted by Jun Zhu on Sat, 10/01/2022 - 09:34
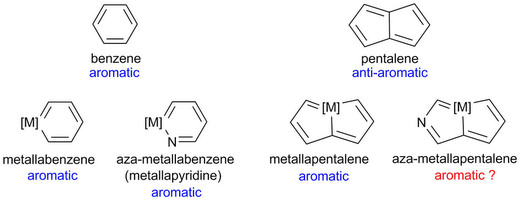
Metallaaromatics as a new class of organometallic compounds have attracted considerable attention in recent years. Metallaaromatic compounds where the number of fused rings forming the metalla-polycyclic skeleton exceeds seven have not been reported to date. Likewise, metallaaromatic compounds containing two transition-metal centres are rarely encountered in the literature. In this work two dicationic diosma-octacyclic complexes have successfully been synthesized and fully characterized.