Theoretical study on the interconversion of silabenzenes and their monocyclic non-aromatic isomers via the [1,3]-substituent shift: Interplay of aromaticity and Bent's rule
Submitted by Jun Zhu on Fri, 08/29/2014 - 09:47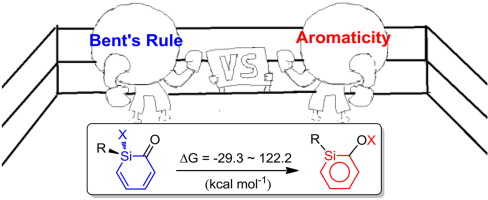
Density functional theory (DFT) calculations were performed to examine the substituent effects on the interconversion of silabenzenes and their monocyclic non-aromatic isomers. A previous study suggested that aromaticity is the driving force for this process. Interestingly, our systematic calculations reveal that the contribution from aromaticity can be evaluated quantitatively (ca. 30 kcal mol-1). Thus it is the interplay of aromaticity and Bent's rule that determine their relative stabilities.
