σ-Aromaticity in an Unsaturated Ring: Osmapentalene Derivatives Containing a Metallacyclopropene Unit
Submitted by Jun Zhu on Wed, 02/04/2015 - 19:29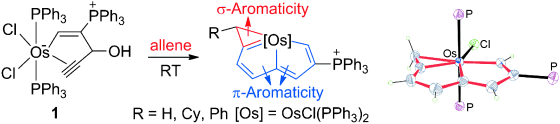
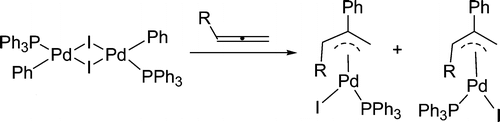
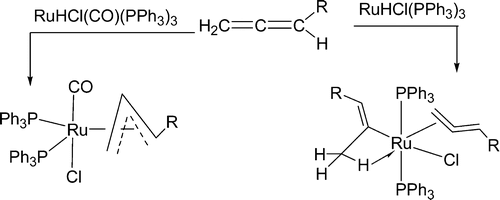
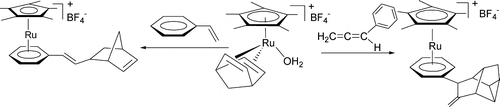
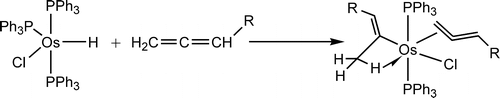
In general, aromaticity can be clarified as π- and σ-aromaticity according to the type of electrons with major contributions. The traditional π-aromaticity generally describes the π-conjugation in fully unsaturated rings whereas σ-aromaticity may stabilize fully saturated rings with delocalization caused by σ-electron conjugation. Reported herein is an example of σ-aromaticity in an unsaturated three-membered ring (3 MR), which is supported by experimental observations and theoretical calculations.