Hyperconjugative aromaticity and protodeauration reactivity of polyaurated indoliums
Submitted by Jun Zhu on Thu, 12/19/2019 - 02:09
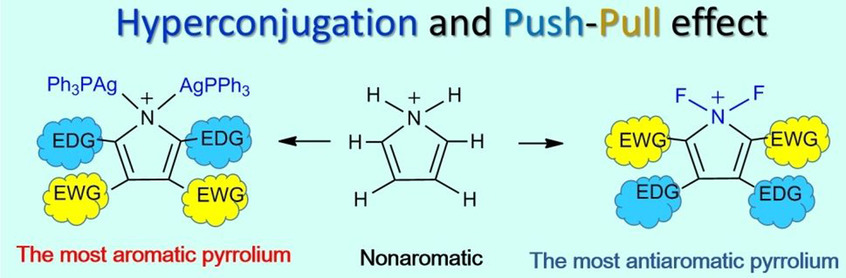
Aromaticity generally describes a cyclic structure composed of sp2-hybridized carbon or hetero atoms with remarkable stability and unique reactivity. The doping of even one sp3-hybridized atom often damages the aromaticity due to the interrupted electron conjugation. Here we demonstrate the occurrence of an extended hyperconjugative aromaticity (EHA) in a metalated indole ring which contains two gem-diaurated tetrahedral carbon atoms. The EHA-involved penta-aurated indolium shows extended electron conjugation because of dual hyperconjugation.