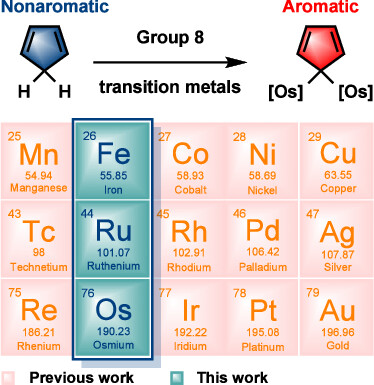
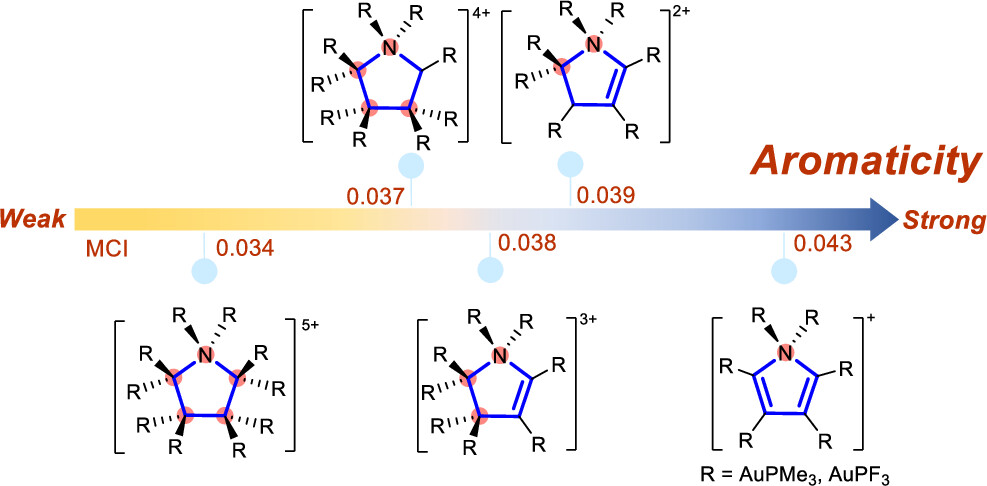
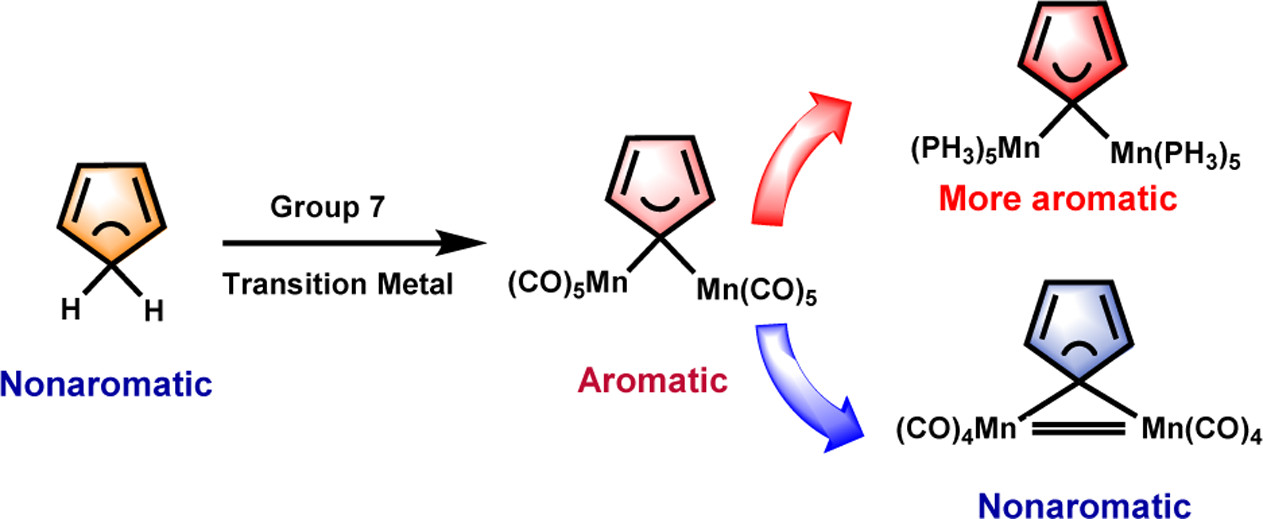
Probing hyperconjugative aromaticity of cyclopentadiene and pyrroliums containing groups 13, 15, and 16 substituents
Submitted by Jun Zhu on Thu, 01/16/2025 - 20:50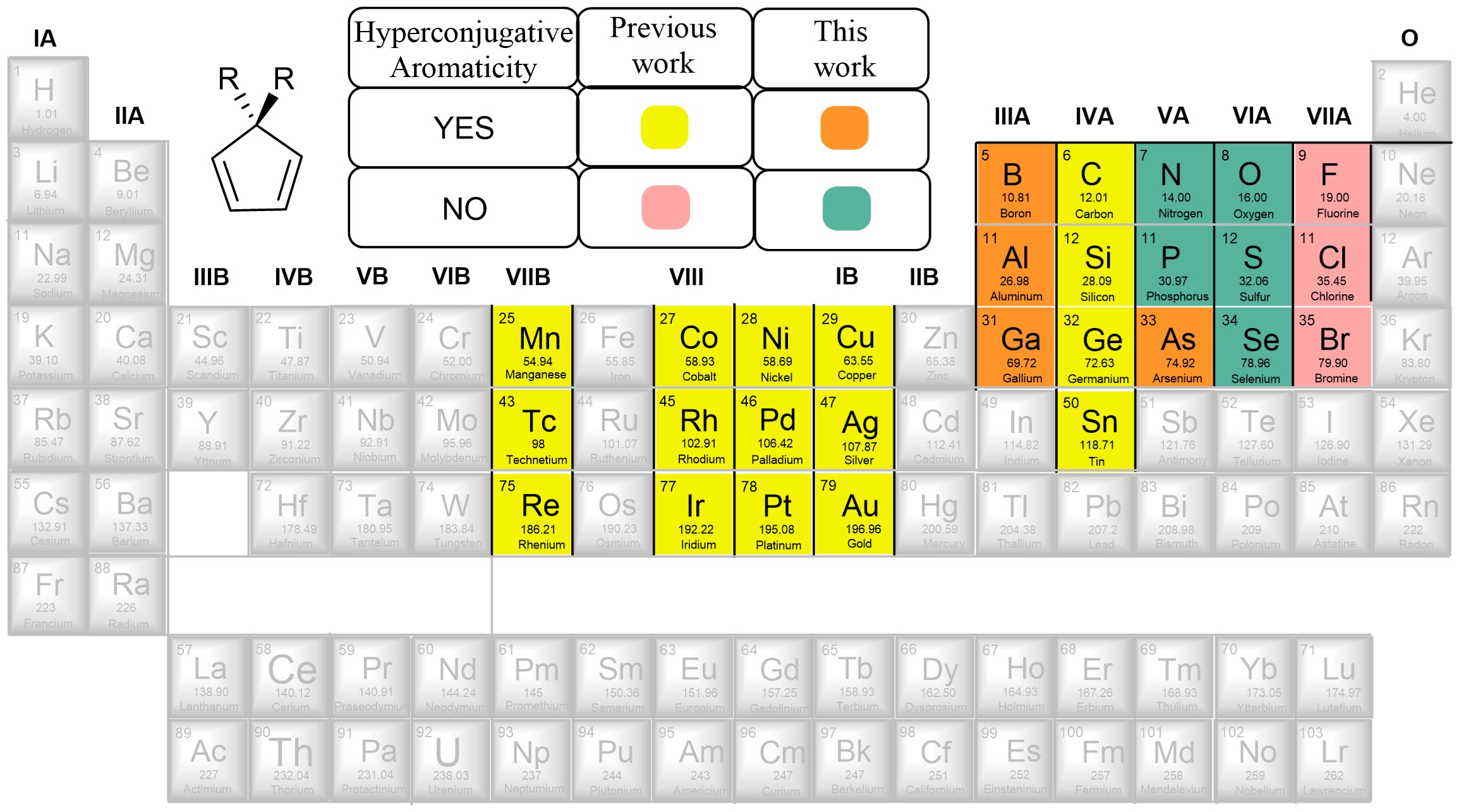
Combining aromaticity and hyperconjugation, two important concepts in organic chemistry, leads to hyperconjugative aromaticity, which was first proposed by Mulliken in 1939. However, previous studies on hyperconjugative aromaticity have mainly focused on substituents containing either main-group elements (group 14) or transition metals in groups 7, 9, 10, and 11. In this study, we perform density functional theory (DFT) calculations on cyclopentadiene and pyrrolium derivatives containing groups 13, 15 and 16 substituents to examine the possibility of achieving hyperconjugative aromaticity.