NO-modulated triplet ground state and adaptive antiaromaticity in BN-doped cyclobutadienes: A combined DFT and machine learning study
Submitted by Jun Zhu on Mon, 10/13/2025 - 16:02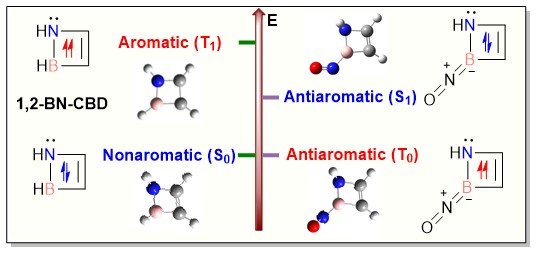
Controlling aromaticity across electronic states is crucial for designing novel species. While aromaticity typically could be achieved in either the lowest singlet state (S0) or the lowest triplet state (T₁), dual-state aromaticity or antiaromaticity remains less developed. Herein, we demonstrate that NO-substitution uniquely induces antiaromaticity in both S0 and T1 states of 1,2-BN-doped cyclobutadiene (1,2-BN-CBD), initially nonaromatic in S0 and weakly aromatic in T1.

