Craig-Type Möbius Aromaticity and Antiaromaticity in Dimetalla[10]annulenes: A Metal-Induced Yin-and-Yang Pair
Submitted by Jun Zhu on Wed, 08/23/2017 - 10:06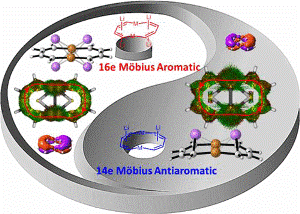
Aromaticity, one of the most fundamental concepts in chemistry, can be classified as Hückel- and Möbius-type according to the electron count and topology. In comparison with numerous Hückel aromatics containing 4n+2 π-electrons, Möbius aromatics with 4n π-electrons, especially the Craig-type species are particularly limited.