Adaptive aromaticity in ruthenacycles
Submitted by Jun Zhu on Mon, 03/16/2020 - 09:21
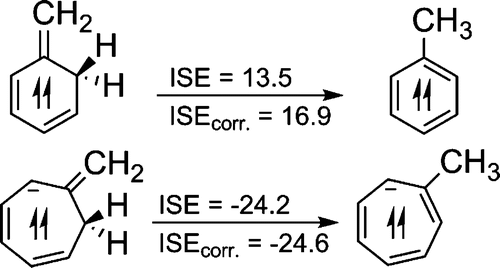
Adaptive aromaticity in the lowest singlet and triplet states is a rare property found among molecular systems. So far, only osmapentalene and osmapyridinium have been found to possess the adaptive aromaticity. Although it has been confirmed that the pattern of electron excitation is a key factor to achieve the adaptive aromaticity, further investigation of the metal center effect has not yet been made. Ruthenium, another Group 8 transition metal, can form metallacycles similar to the osmium counterparts.