Probing the reactivity of microhydrated α-nucleophile in the anionic gas-phase SN2 reaction
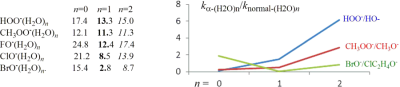
To probe the kinetic performance of microsolvated α-nucleophile, the G2(+)M calculations were carried out for the gas-phase SN2 reactions of monohydrated and dihydrated α-oxy-nucleophiles XO−(H2O)n = 1,2 (X = HO, CH3O, F, Cl, Br), and α-sulfur-nucleophile, HSS−(H2O)n = 1,2, toward CH3Cl. We compared the reactivities of hydrated α-nucleophiles to those of hydrated normal nucleophiles. Our calculations show that the α-effect of monohydrated and dihydrated α-oxy-nucleophiles will become weaker than those of unhydrated ones if we apply a plot of activation barrier as a function of anion basicity. Whereas the enhanced reactivity of monohydrated and dihydrated ROO− (R = H, Me) could be observed if compared them with the specific normal nucleophiles, RO− (R = H, Me). This phenomena can not be seen in the comparisons of XO−(H2O)n = 1,2 (X = F, Cl, Br) with ClC2H4O−(H2O)n = 1,2, a normal nucleophile with similar gas basicity to XO−(H2O)n = 1,2. These results have been carefully analyzed by natural bond orbital theory and activation strain model. Meanwhile, the relationships between activation barriers with reaction energies and the ionization energies of α-nucleophile are also discussed.
http://onlinelibrary.wiley.com/doi/10.1002/jcc.23862/abstract
