Trans Influence of Boryl Ligands and Comparison with C, Si, and Sn Ligands
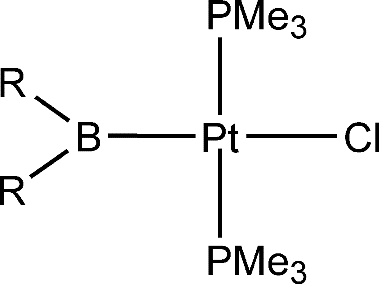
In this paper, the trans influence of boryl ligands, together with that of other ligands commonly believed to have a strong trans influence, has been investigated theoretically via density functional theory (DFT) calculations on a series of square-planar platinum(II) complexes of the form trans-[PtL(Cl) (PMe3)(2)]. The following order of trans influence has been obtained: -BMe2 > -SiMe3 > -BH2 > -SnMe3 - -BNHCH2CH(2)NH > -Bpin > -BOCH2CH2O > -BOCH=CHO similar to -Bcat similar to -BCl2 similar to -BBr2 similar to -SiH3 > -CH2CH3 > -CH=CH2 > -H similar to -Me > -C6H5 > -SiCl3 > -SnCl3 > -C-equivalent to CH. Natural bond order analyses have been used to understand how the substituents at the boron center affect the trans-influence properties of the boryl ligands. The major factor is the a-donor strength of the boryl ligand. However, surprisingly, very strong pi acceptors also enhance the trans influence.
