"Sacrificial reagent" strategy for tailoring adaptive aromaticity in pyrrole rings with its application to singlet fission: a combined DFT and machine learning study
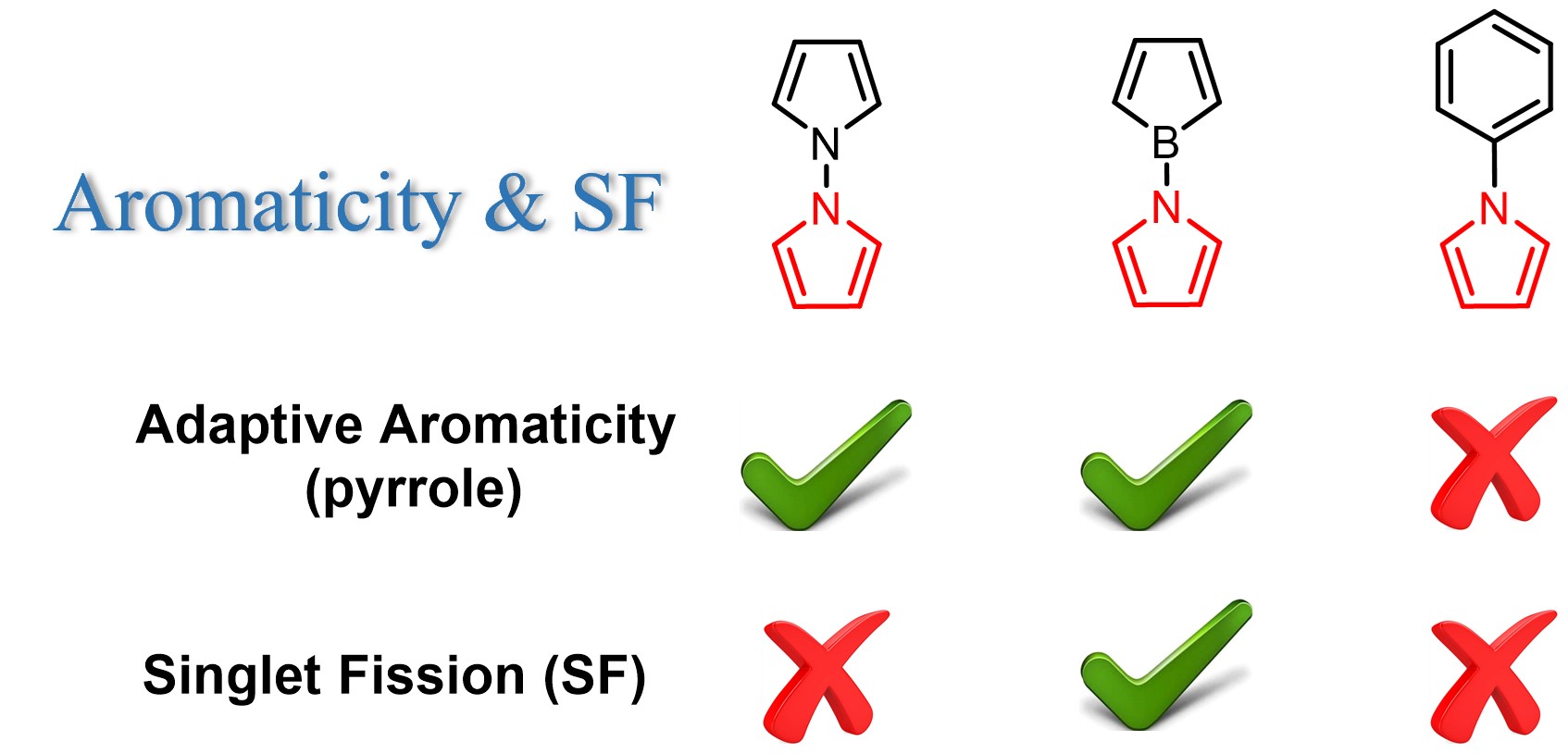
Adaptive aromaticity has attracted substantial attention due to its unconventional two-state aromaticity in both the lowest singlet state (S0) and the lowest triplet state (T1) and its application to the singlet fission. Here we carry out a comprehensive investigation on a series of pyrrole derivatives by density functional theory (DFT) calculations and machine learning. Various aromaticity indices including HOMA, NICS, EDDB, and MCI suggest that the pyrrole rings in these derivatives show adaptive aromaticity when the other ring of bicyclics has weaker (or comparable) aromaticity than the pyrrole ring among these pyrrole derivatives, which could be considered as a sacrificial reagent strategy to achieve adaptive aromaticity of these pyrrole rings. On the contrary, when the other ring such as the phenyl ring has stronger aromaticity than the pyrrole ring, it can not be sacrificed to achieve adaptive aromaticity of the pyrrole rings. More interestingly, a singlet fission chromophore is predicted when the other ring is an antiaromatic borole among these pyrrole derivatives. Additionally, principal component analysis (PCA, one of the most commonly used unsupervised machine learning algorithms) suggests that ΔNICS(1)zz (NICS(1)zz(S0) - NICS(1)zz(T1)) of the other ring in these pyrrole derivatives can be an important factor in predicting E(T1) values, which is also supported by a strong correlation (R2 = 0.98) with between ΔNICS(1)zz and E(T1). All these findings provide an alternative idea for the design of singlet fission chromophores.
https://pubs.rsc.org/en/content/articlelanding/2026/qo/d5qo01255d
