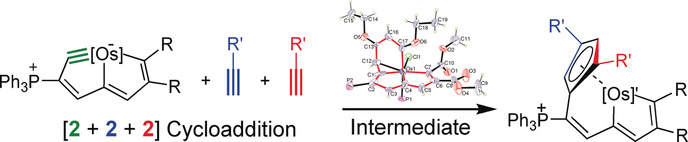
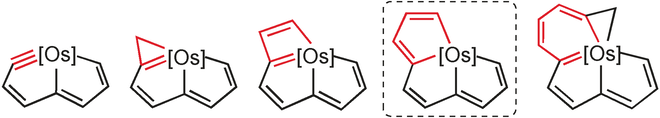
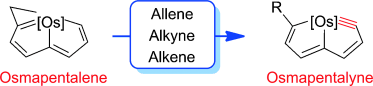
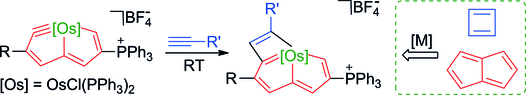
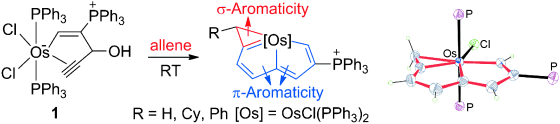
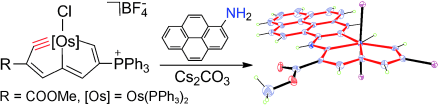
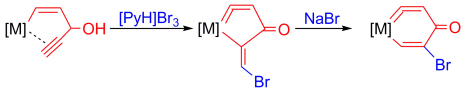
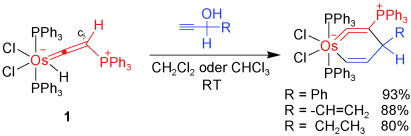
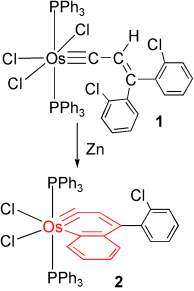
Bowl Inversion in an Exo‐type Supramolecule in the Solid State
Submitted by Jun Zhu on Wed, 08/14/2019 - 09:34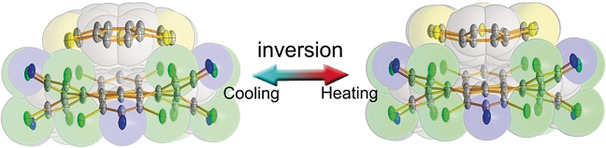
Bowl inversion is a unique property of buckybowls. The polarity and assembly configuration of buckybowls are reversed after bowl inversion. So far, this unique phenomenon has been studied in solution and on surface, but not in solid state due to spatial constraint. Now a series of exo‐type supramolecular assemblies of trithiasumanene and nanographene are investigated. Tuning the electron density of the nanogaphene component was found to directly affect the binding constant of the complex.