Synthesis and Characterization of Osmium Polycyclic Aromatic Complexes via Nucleophilic Reactions of Osmapentalyne
Submitted by Jun Zhu on Mon, 05/22/2017 - 09:18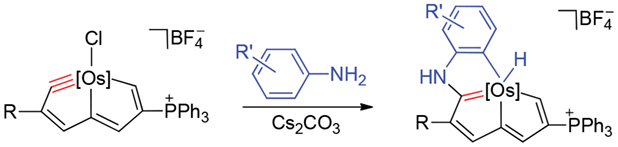
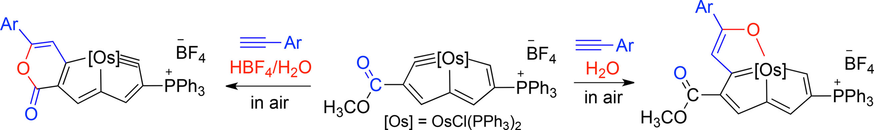
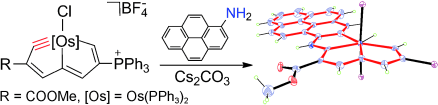
Treatment of osmapentalyne [Os{≡C-C(COOMe)=CH-C=CH-C(PPh3)=CH-}Cl(PPh3)2]+BF4- with arylamines in the presence of Cs2CO3 produced osmium-bridged polycyclic aromatic complexes. In this reaction, metal carbyne of osmapentalyne was first attacked by nucleophiles, followed by a C-H oxidative addition. The UV-Vis spectra of these osmium-bridged polycyclic aromatic complexes were measured. The result shows that these osmium-bridged polycyclic aromatic complexes have broad absorption in the UV-Vis region up to 650 nm.