Theoretical Investigation of Alkyne Metathesis Catalyzed by W/Mo Alkylidyne Complexes
Submitted by Jun Zhu on Thu, 10/31/2013 - 23:40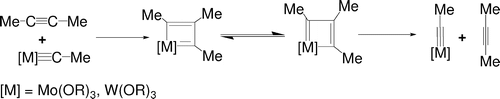
In this paper, the mechanism of alkyne metathesis catalyzed by W/Mo alkylidyne complexes has been theoretically investigated with the aid of density functional theory calculations. Calculations on various model alkylidyne complexes M( CMe)(OR)(3) (M = W, Mo; R = Me, CH2F), W( CMe)(NMe2)(3), and W( CMe)(Cl)(3) allow us to examine the factors that influence the reaction barriers. In the reaction mechanism, metallacyclobutadienes are initially formed from a ring-closing step between alkynes and alkylidyne complexes. A ring-opening step then gives the metathesis products.
