Catalytic hydroboration of aldehydes, ketones, alkynes and alkenes initiated by NaOH
Submitted by Jun Zhu on Mon, 01/29/2018 - 16:32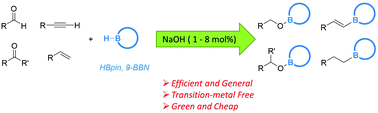
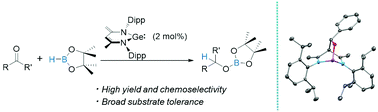
Commercially available NaOH powder is shown to be an efficient transition-metal-free initiator for the catalytic hydroboration of aldehydes, ketones, alkynes and alkenes with HBpin and 9-BBN under mild conditions. Combined experimental and theoretical studies suggest that the catalytically active species is a boron hydride generated in situ from the reaction mixture.
http://pubs.rsc.org/en/content/articlelanding/2017/gc/c7gc01632h#!divAbstract