Adaptive Aromaticity in Metallasilapentalynes
Submitted by Jun Zhu on Fri, 03/19/2021 - 09:18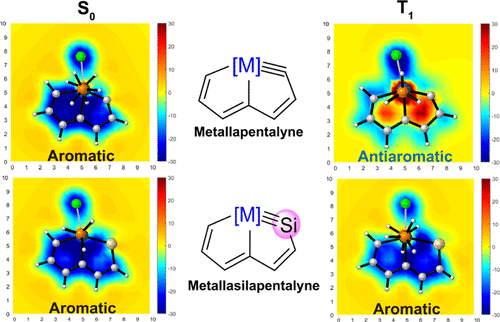
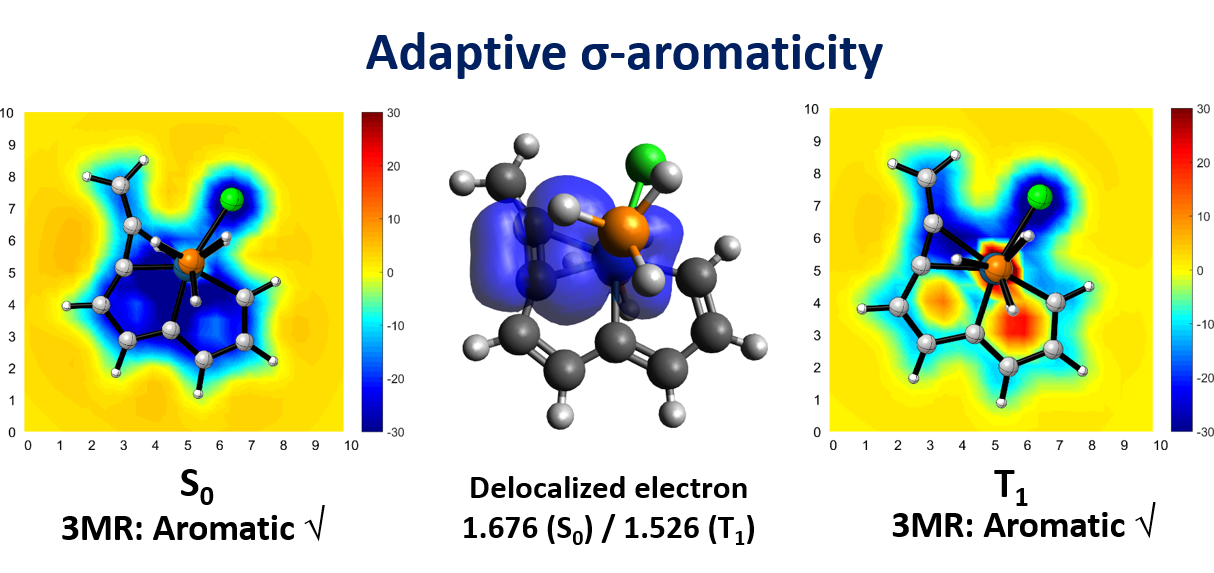
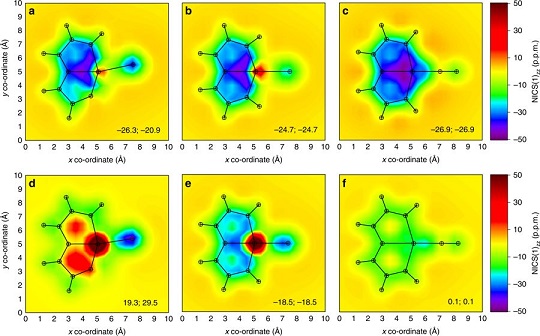
Cyclic molecules with 4n + 2 or 4n electrons are aromatic in the lowest singlet state (S0) or the lowest triplet state (T1) according to Hückel and Baird’s rules. Thus, the design of aromatic species in both the S0 and T1 states (termed as adaptive aromaticity) is particularly challenging. In this work, we demonstrate that metallasilapentalynes show adaptive aromaticity supported by structural, magnetic, and electronic indices, in sharp contrast to metallapentalynes, which exhibit aromaticity in the S0 state only.