Electrochemical Migratory Cyclization of N-acylsulfonamides
Submitted by Jun Zhu on Tue, 05/24/2022 - 22:33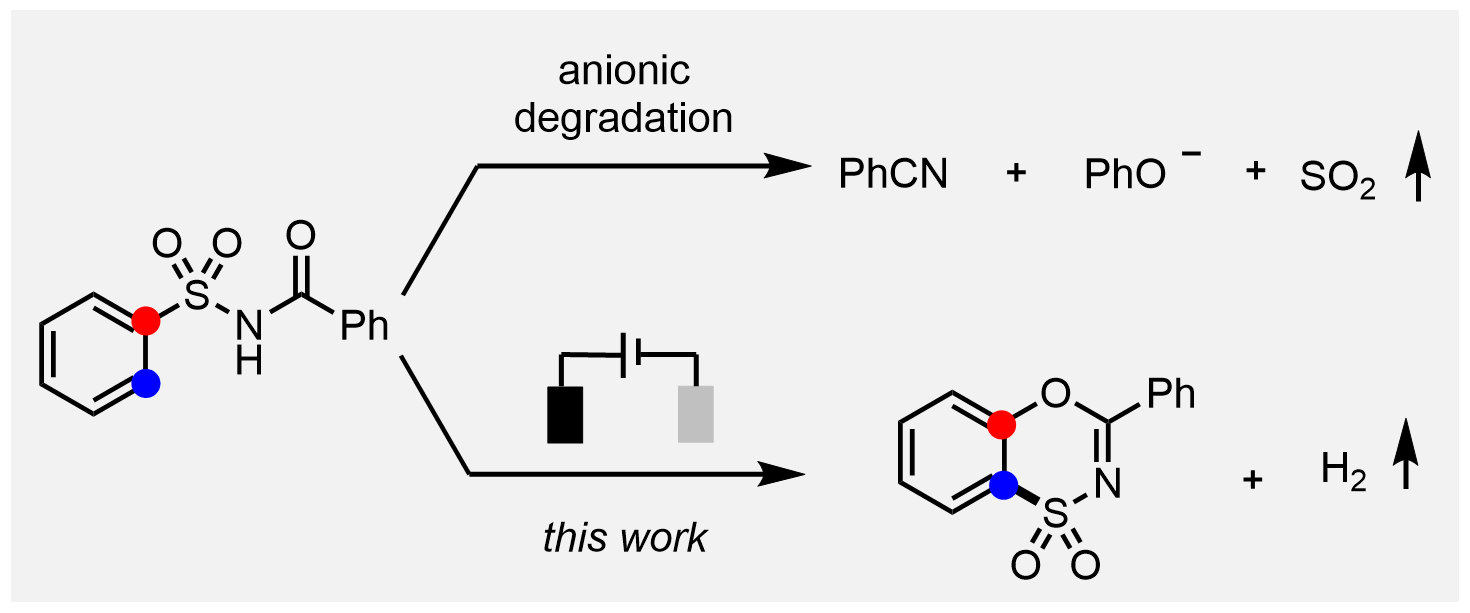
Benzoxathiazine dioxide, as a bioisostere of the clinically widely used diazoxide, exhibits interesting biological activity. However, limited success has been achieved in terms of its concise and direct synthesis. We report herein a facile electrochemical migratory cyclization of N -acylsulfonamides to access a diverse array of benzoxathiazine dioxides. The inclusion of electrochemistry is crucial for realizing such a novel transformation, which is substantiated both by the experiments and density-functional-theory calculations.
