Stabilizing a 20-Electron Metallaazulyne by Aromaticity
Submitted by Jun Zhu on Sun, 06/26/2022 - 08:12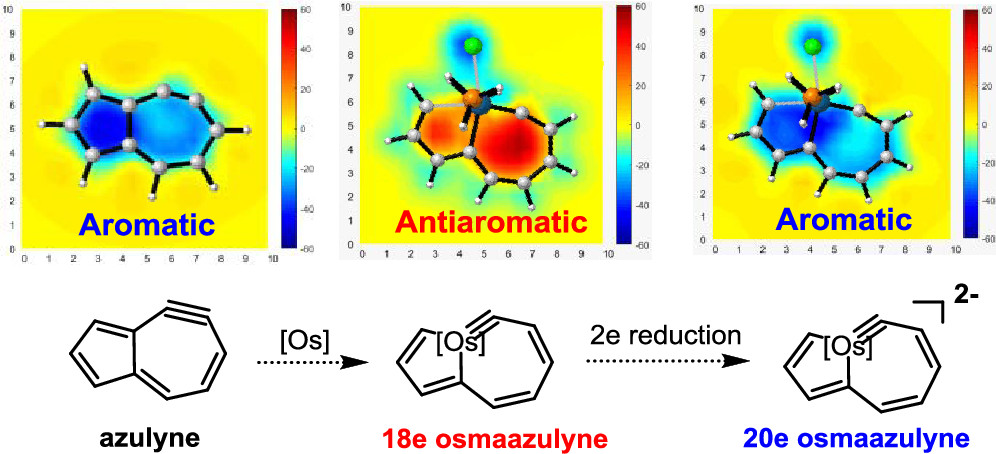
The 18-electron rule states that metal complexes with 18 valence electron metal centers are thermodynamically stable because nine valence orbitals of transition metals including one s orbital, three p orbitals, and five d orbitals can collectively accommodate 18 electrons, achieving the same electron configuration as the noble gas in the period. Thus, 20-electron compounds are extremely rare due to a violation of such a rule.
