Stabilizing a 20-Electron Metallaazulyne by Aromaticity
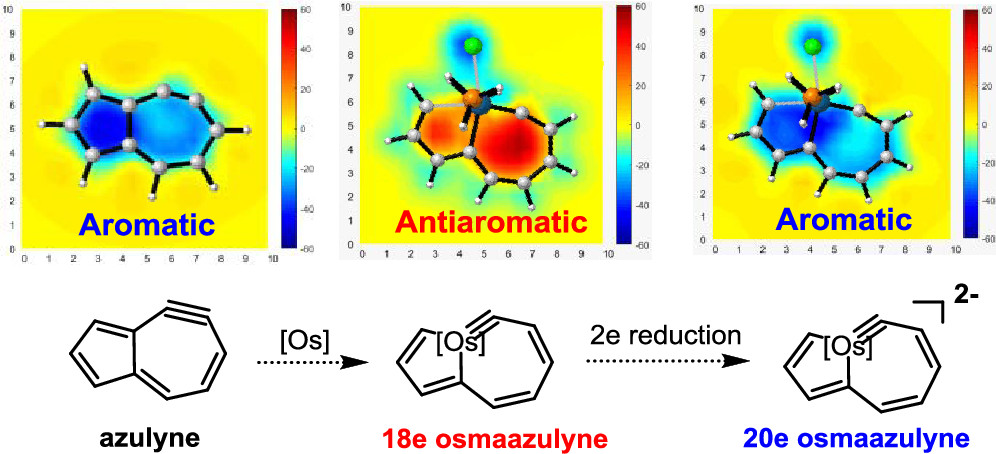
The 18-electron rule states that metal complexes with 18 valence electron metal centers are thermodynamically stable because nine valence orbitals of transition metals including one s orbital, three p orbitals, and five d orbitals can collectively accommodate 18 electrons, achieving the same electron configuration as the noble gas in the period. Thus, 20-electron compounds are extremely rare due to a violation of such a rule. Here, we demonstrate a 20-electron metallaazulyne via density functional theory calculations stabilized by aromaticity, which was supported by various aromaticity indices including nucleus-independent chemical shift, anisotropy of the induced current density, the isochemical shielding surface, and electron density of delocalized bonds. Interestingly, when a transition metal fragment is first introduced into the aromatic azulyne molecule, the resulting osmaazulyne becomes antiaromatic, in sharp contrast to the previous transformation from pentalyne to metallapentalyne. More interestingly, when osmaazulyne is reduced by two electrons, the resulting 20e osmaazulyne becomes aromatic. Our findings highlight an important application of aromaticity in stabilizing 20e species, inviting experimental verification.
