Synthesis and Reactivity of an Anti-van’t Hoff/Le Bel Compound with a Planar Tetracoordinate Silicon(II) Atom
Submitted by Jun Zhu on Thu, 03/23/2023 - 09:40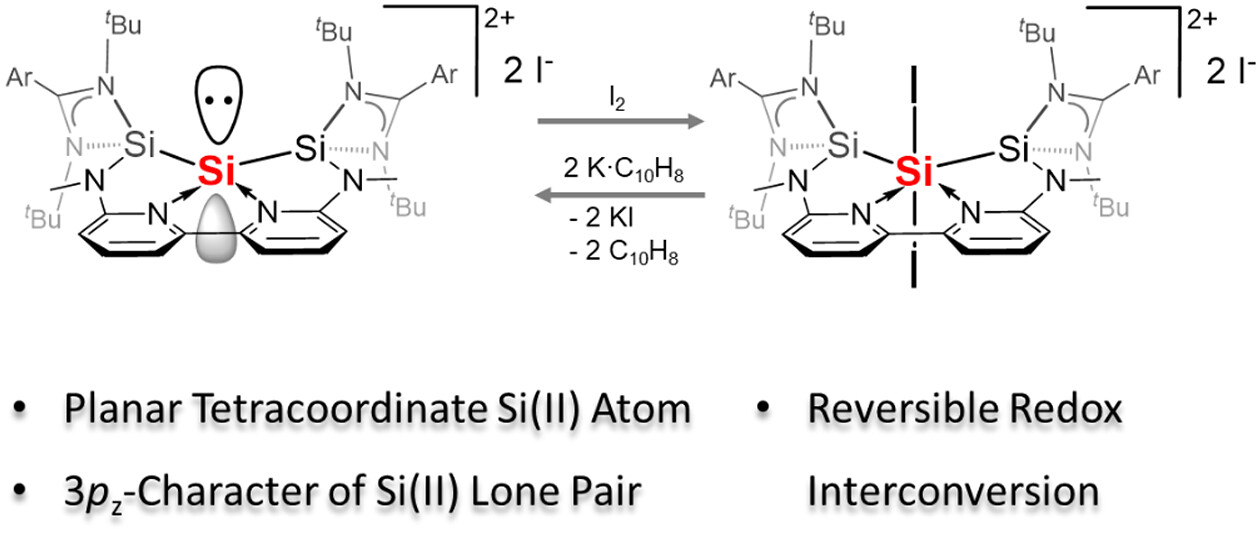
For a long time, planar tetracoordinate carbon (ptC) represented an exotic coordination mode in organic and organometallic chemistry, but it is now a useful synthetic building block. In contrast, realization of planar tetracoordinate silicon (ptSi), a heavier analogue of ptC, is still challenging. Herein we report the successful synthesis and unusual reactivity of the first ptSi species of divalent silicon present in 3, supported by the chelating bis(N-heterocyclic silylene)bipyridine ligand, 2,2′-{[(4-tBuPh)C(NtBu)]2SiNMe}2(C5N)2, 1].