Silicon-Containing Formal 4 pi-Electron Four-Membered Ring Systems: Antiaromatic, Aromatic, or Nonaromatic?
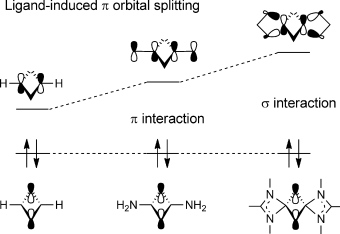
Density functional theory calculations (B3LYP) have been carried out to investigate the 4π-electron systems of 2,4-disila-1,3-diphosphacyclobutadiene (compound 1) and the tetrasilacyclobutadiene dication (compound 2). The calculated nucleus-independent chemical shift (NICS) values for these two compounds are negative, which indicates that the core rings of compounds 1 and 2 have a certain amount of aromaticity. However, deep electronic analysis reveals that neither of these two formal 4π-electron four-membered ring systems is aromatic. Compound 1 has very weak, almost negligible antiaromaticity, and the amidinate ligands attached to the Si atoms play an important role in stabilizing this conjugated 4π-electron system. The monoanionic bidentate ligand interacts with the conjugated π system to cause π-orbital splitting. This ligand-induced π-orbital splitting effect provides an opportunity to manipulate the gap between occupied and unoccupied π orbitals in conjugated systems. Conversely, compound 2 is nonaromatic because its core ring does not have a conjugated π ring system and does not fulfill the requirements of a Hückel system.
