Reactions of Hydrotris(pyrazolyl)borate (Tp)-Supported Ruthenium Dihydrogen Complexes [TpRu(L2)(H2)]+ (L2 = dppm, dppp, (PPh3)2) with O2
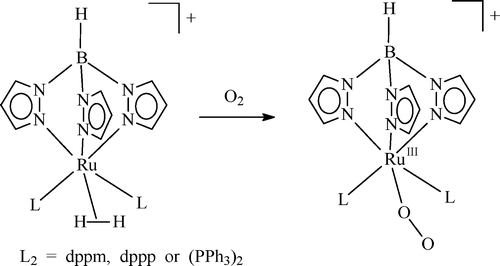
The η2-dihydrogen complex [TpRu(L2)(H2)]+ (L2 = dppm, dppp, or (PPh3)2) prepared in situ by protonation of the hydride precursor reacts with O2 to yield the paramagnetic RuIII-superoxo complex [TpRuIII(L2)(O2)]+, in which antiferromagnetic coupling between the RuIII ion (d5, S = 1/2) and the coordinated superoxide radical (S = 1/2) does not seem to be present. In THF, the superoxo moiety of the complex readily abstracts a hydrogen atom from the solvent to generate the hydroperoxo (−OOH-) group, which then changes into the hydroxo ligand by transferring an oxygen atom to a phosphine ligand. Density functional theory calculation at the B3LYP level on the model complex [TpRu(PH3)2(O2)]+ shows that the RuIII-superoxo(O2-) structure with a triplet state is more stable than the RuIV-peroxo(O22-) structure with a singlet state by 5.2 kcal/mol. On the other hand, the analogous Cp model complex [CpRu(PH3)2(O2)]+ prefers the RuIV-peroxo(O22-) structure over the RuIII-superoxo(O2-) structure by 2.9 kcal/mol.
