Mechanistic Insight into the Ni-Catalyzed Kumada Cross-Coupling: Alkylmagnesium Halide Promotes C–F Bond Activation and Electron-Deficient Metal Center Slows Down β-H Elimination
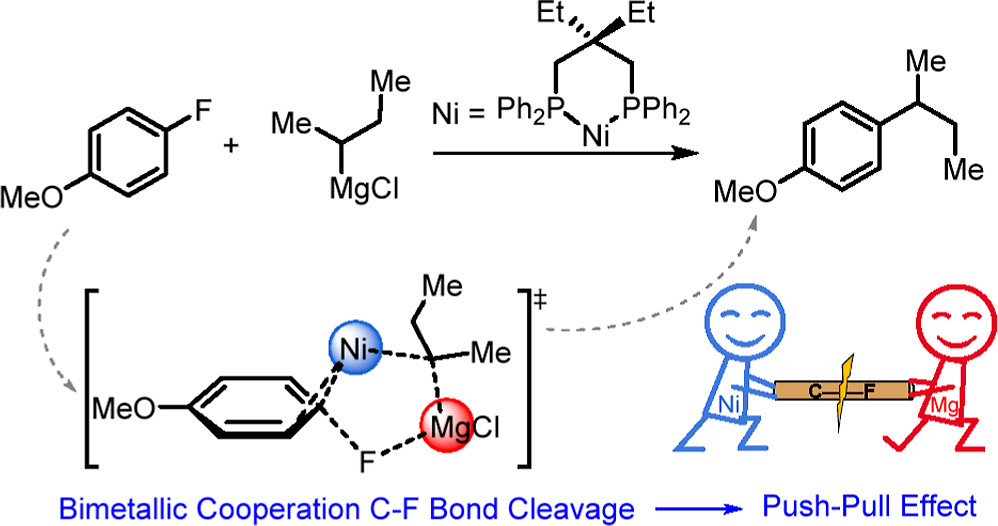
The Ni-catalyzed Kumada–Tamao–Corriu (KTC) cross-coupling between aryl fluorides and alkyl Grignard reagents has been used to achieve a highly selective Csp2–Csp3 bond construction via the carbon–fluorine (C–F) bond activation. However, the detailed mechanism of this groundbreaking KTC reaction remains unclear. Herein, we perform a series of analyses by density functional theory (DFT) calculations in order to understand the reaction mechanisms for the selective activation of a highly inert C–F bond by Ni catalysts with bidentate phosphorus ligands. An alternative mechanism for Ni/Mg bimetallic cooperation C–F bond cleavage instead of a traditional oxidative addition was proposed. The push–pull interaction in the transition state provided by the Ni center and the Lewis acid of the Mg cation smoothly breaks the C–F bond, supported by the significantly decreased activation energy from 30.9 to 4.6 kcal mol–1 and principal interacting orbital analysis. Owing to the elevated lowest unoccupied molecular orbital energy level and the electron-deficient metal center caused by the bidentate phosphorus ligand, the β-H elimination could be impeded, increasing the selectivity of KTC cross-coupling. Our DFT results rationally explain the experimental observations, which will be helpful for further development of KTC cross-coupling.
