Predicting Dinitrogen Activation and Coupling with Carbon Dioxide and Other Small Molecules by Methyleneborane: A Combined DFT and Machine Learning Study
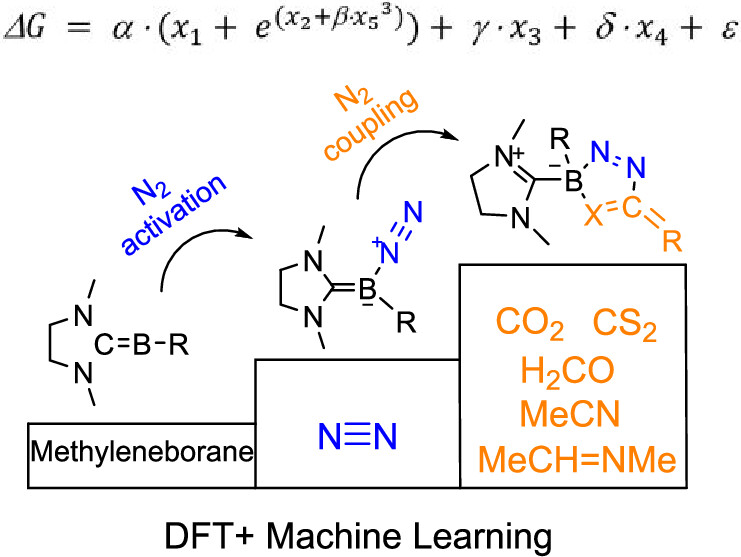
The capture of carbon dioxide is extremely important due to the increasingly severe greenhouse effect, and the conversion of dinitrogen into high-value N–C compounds is of great significance. Here, we predict through density functional theory calculations that the coupling of dinitrogen with carbon dioxide by methyleneborane becomes favorable both thermodynamically and kinetically. Machine learning analysis suggests that increasing the HOMO–LUMO gap or the charge on the boron atom or decreasing the charge of the nitrogen atom will reduce the reaction energies. N2 coupling is examined as a concerted step, as CO2 is a σ donor and a π acceptor. Besides, N2 coupling with other small molecules including formaldehyde, sulfur dioxide, acetonitrile, and N-ethylidenemethylamine was also examined in both thermodynamics and kinetics. Our findings highlight a crucial role of main group species in N2 coupling chemistry.
