Switchable Reactivities of Metalated Phosphasilenes Regulated by Reversible 1,2-Metal Migration
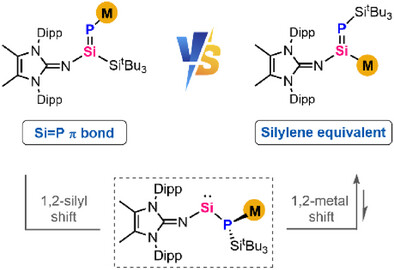
Anionic reagents with silicon-containing double bonds, M(R)Si═ERn (E = main group elements), have garnered significant interest owing to their unique metal-mediated reactivity and their potential in transferring the Si═E unit. Within this domain, the intriguing field of Si-metalated phosphasilenes remains uncharted. Herein, we present a novel strategy for the efficient synthesis of P-metalated phosphasilenes and their subsequent transformation into Si-metalated phosphasilenes via a distinctive phosphinosilylene intermediate formed during a metal-mediated skeletal rearrangement. While P-metalated phosphasilenes exhibit a pronounced π-bonding character in the Si═P linkage, the skeletal rearrangement attenuates both the Si═P and Si─M bonds in the resulting Si-metalated phosphasilenes, rendering them effective silylene equivalents. Moreover, formal transmetallation of Si-metalated phosphasilene with dimetal carbonyl complexes provides access to a broader array of Si-metalated analogues. This transmetallation proceeds through the phosphinosilylene intermediate, as evidenced by the isolation of a silylene–dimanganese complex, thereby revealing an uncharted mechanistic manifold for this class of transformations.
