Antiaromaticity-Promoted Activation of Dihydrogen with Borole Fused Cyclooctatetraene Frustrated Lewis Pairs: A Density Functional Theory Study
Submitted by Jun Zhu on Mon, 07/13/2020 - 19:39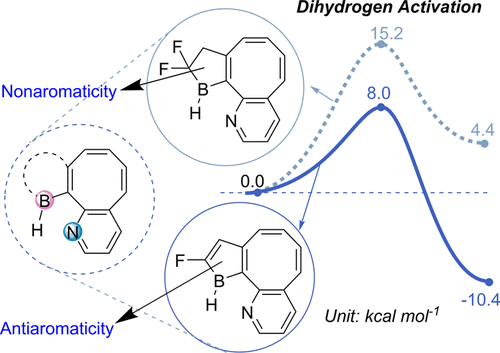
Aromaticity and frustrated Lewis pairs (FLP), two important concepts in chemistry, have attracted considerable attention from theoretical and experimental chemists. However, combining these two concepts together for H2 activation is less developed. Herein, we report a density functional theory study on antiaromaticity-promoted H2 splitting. The antiaromatic borole (as Lewis acid) and aromatic pyridine (as Lewis base) were introduced into the cyclooctetraene skeleton. Due to the geometric constraints, such systems can be classified as FLPs.