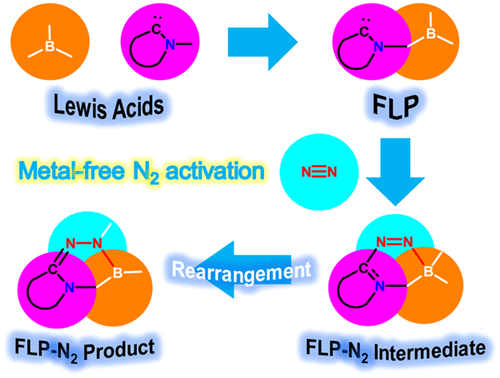
Understanding reaction mechanisms of metal-free dinitrogen activation by methyleneboranes
Submitted by Jun Zhu on Fri, 08/26/2022 - 21:21
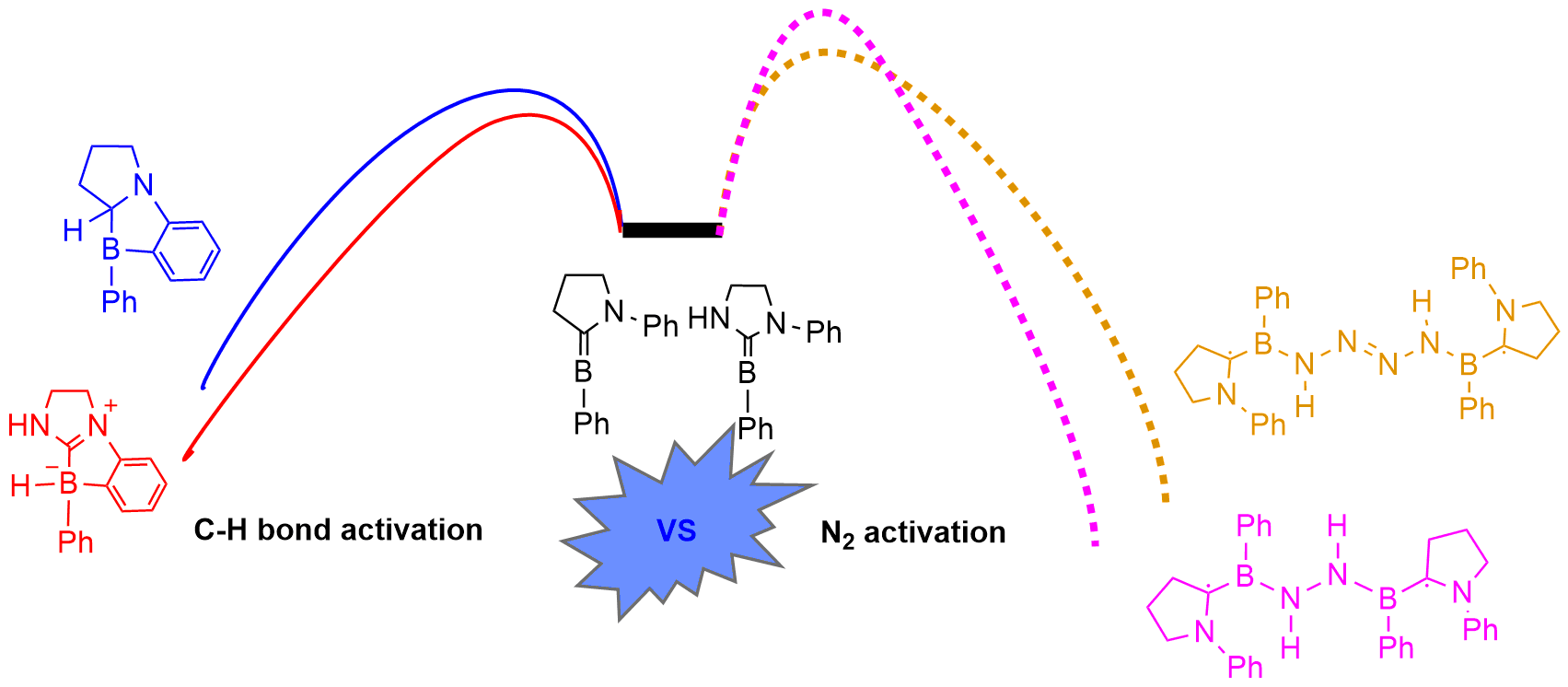
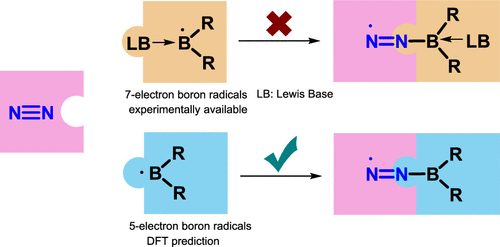
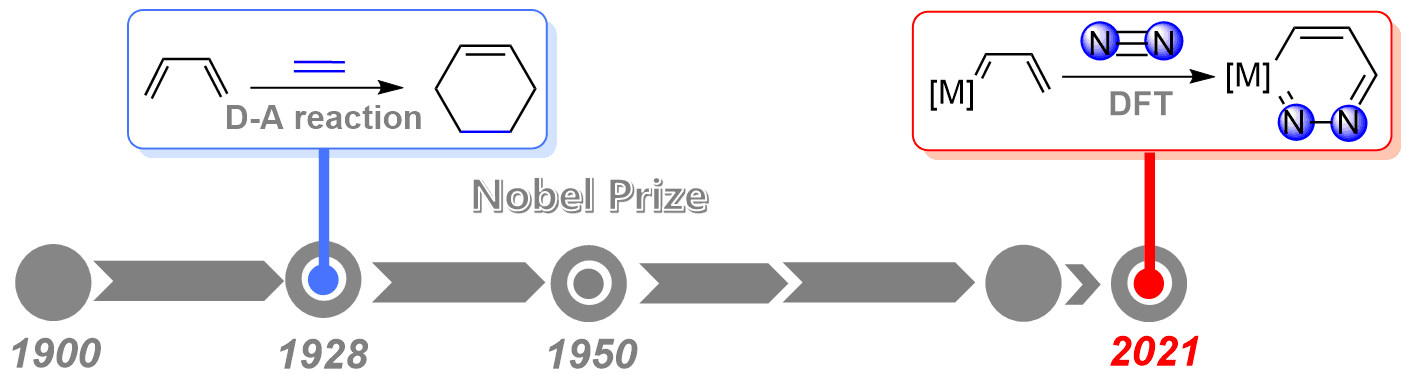
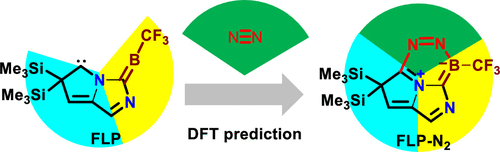
Dinitrogen activation under mild conditions is important but extremely challenging due to the inert nature of the N-N triple bond evidenced by high bond dissociation energy (945 kJ/mol) and large HOMO-LUMO gap (10.8 eV). In comparison with largely developed transition metal systems, the reported main group species on dinitrogen activation are rare. Here, we carry out density functional theory calculations on methyleneboranes to understand the reaction mechanisms of their dinitrogen activation.