Probing the origin of the stereoselectivity and enantioselectivity of cobalt-catalyzed [2 + 2] cyclization of ethylene and enynes
Submitted by Jun Zhu on Sun, 02/21/2021 - 10:19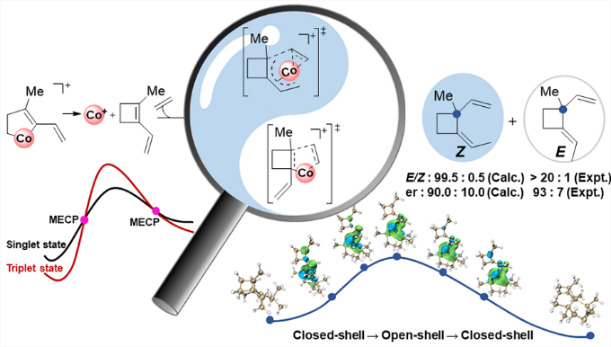
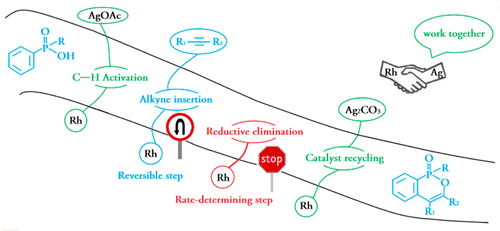
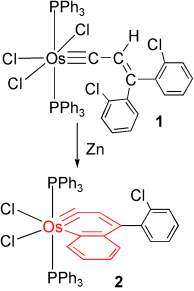
The cyclobutane unit is important to prepare complex natural products with biological activity due to the high ring strain. Among various approaches, [2 + 2] cycloaddition is one of the major strategies to prepare cyclobutane under light conditions. Recently, Rajanbabu's group reported tandem catalysis for asymmetric coupling of inactivated ethylene and enynes to functionalized cyclobutenes or cyclobutanes. However, the reaction mechanisms remain unproven.