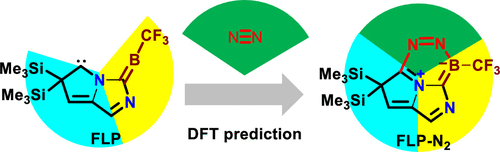
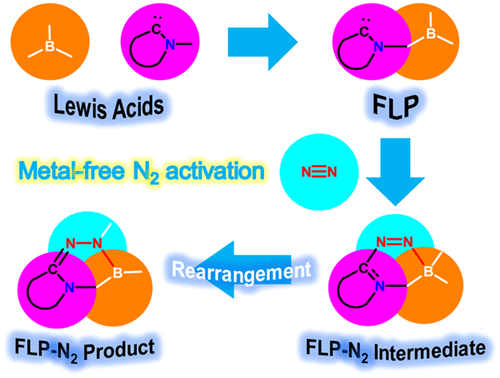
Predicting Dinitrogen Activation by Five-Electron Boron-Centered Radicals
Submitted by Jun Zhu on Sat, 01/29/2022 - 19:36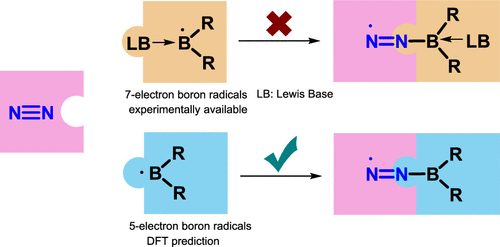
Due to the high bond dissociation energy (945 kJ mol–1) and the large highest occupied molecular orbital–lowest unoccupied molecular orbital (HOMO–LUMO) gap (10.8 eV), dinitrogen activation under mild conditions is extremely challenging. On the other hand, the conventional Haber–Bosch ammonia synthesis under harsh conditions consumes more than 1% of the world’s annual energy supply. Thus, it is important and urgent to develop an alternative approach for dinitrogen activation under mild conditions.