Probing the Origin of Ambiphilic Reactivity in Osmapentalyne Complexes: Interplay of Ring Strain, Aromaticity, and Phosphonium Substituent
Submitted by Jun Zhu on Sun, 05/09/2021 - 21:32
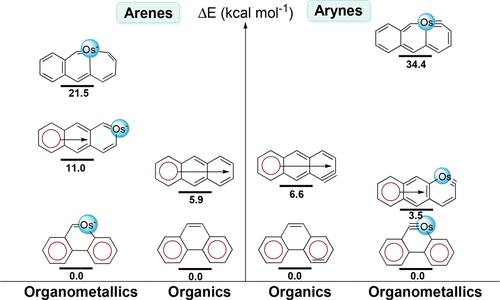
Ambiphilic reactivity is a fascinating topic in chemical reactions, attracting considerable interest because ambiphilic reagents can display properties of both nucleophilicity and electrophilicity. However, most of the previous attention has been focused on the characterization of the ambiphilic reactivity, whereas the origin is less understood. Here we carry out thorough density functional theory (DFT) calculations to probe the origin of the ambiphilic reactivity of the carbyne atom in osmapentalynes, observed previously in experiment.