σ Aromaticity Dominates in the Unsaturated Three-Membered Ring of Cyclopropametallapentalenes from Groups 7–9: A DFT Study
Submitted by Jun Zhu on Tue, 11/17/2015 - 15:49
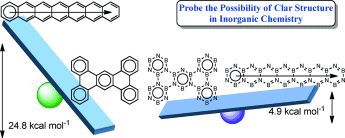
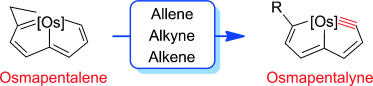
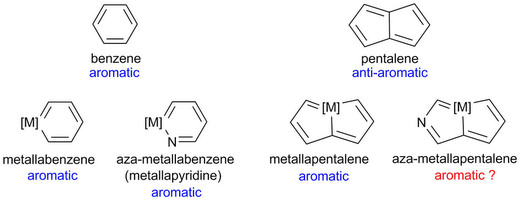
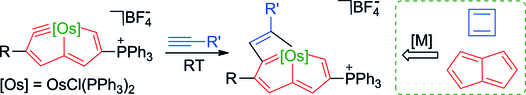
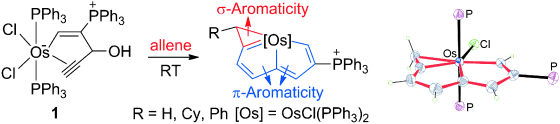
Aromaticity, an old but still fantastic topic, has long attracted considerable interest of chemists. Generally, π aromaticity is described by π-electron delocalization in closed circuits of unsaturated compounds whereas σ-electron delocalization in saturated rings leads to σ aromaticity. Interestingly, our recent study shows that σ aromaticity can be dominating in an unsaturated three-membered ring (3MR) of cyclopropaosmapentalene. An interesting question is raised: Can the σ aromaticity, which is dominant in the unsaturated 3MR, be extended to other cyclopropametallapentalenes?