Theoretical study on the stability and aromaticity in silapentafulvenes towards triplet ground state species
Submitted by Jun Zhu on Fri, 02/28/2020 - 15:26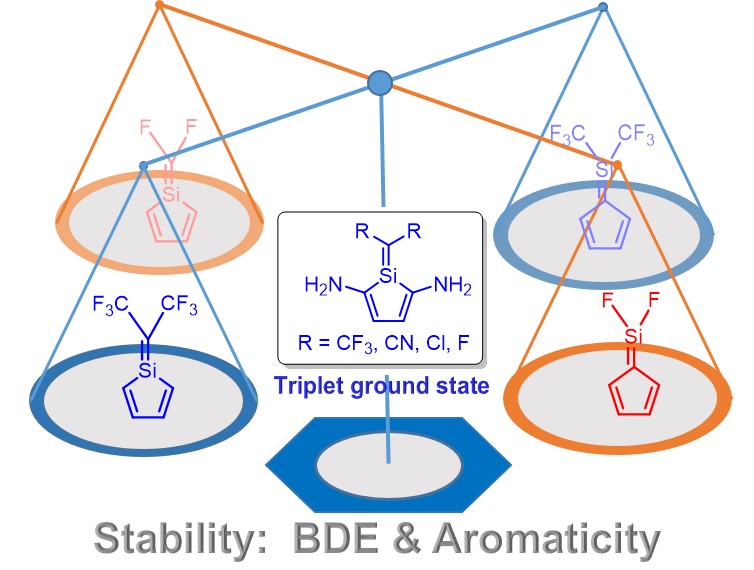
Pentafulvenes are dipolar hydrocarbons since they shift their π-electrons to achieve Hückel aromaticity and thus the electron donating groups at the exocyclic position can enhance their aromaticity. Silapentafulvenes are analogues of pentafulvene formed by the replacement of the carbon atoms at the exocyclic CC double bond with a silicon atom in pentafulvene. It remains unclear how the aromaticity of 5-silapentafulvenes and 6-silapentafulvenes can be changed due to the polarization of the CSi double bond.