Mechanism, Reactivity, and Selectivity in Rh(III)-Catalyzed Phosphoryl-Directed Oxidative C–H Activation/Cyclization: A DFT Study
Submitted by Jun Zhu on Sat, 05/24/2014 - 00:12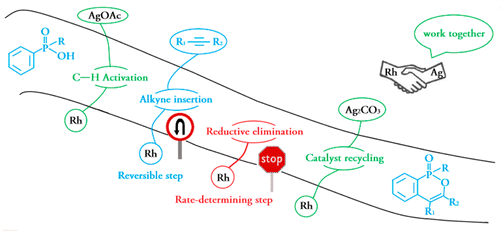
Density functional theory calculations (DFT) have been performed on Rh(III)-catalyzed phosphoryl-directed oxidative C–H activation/cyclization to investigate the detailed mechanism, including four basic steps: C–H activation, alkyne insertion, reductive elimination, and catalyst recycling, each of which consists of different steps. Interestingly, the Rh(III)–AgOAc catalyst system was found to be more favorable in the C–H activation step in comparison with the Rh(III)–Ag2CO3 system, whereas the Rh(I)–Ag2CO3 catalyst system was more efficient for catalyst recycling.